New publication in 2022: Chemical Engineering Journal (IF: 16.744 )
Abstract
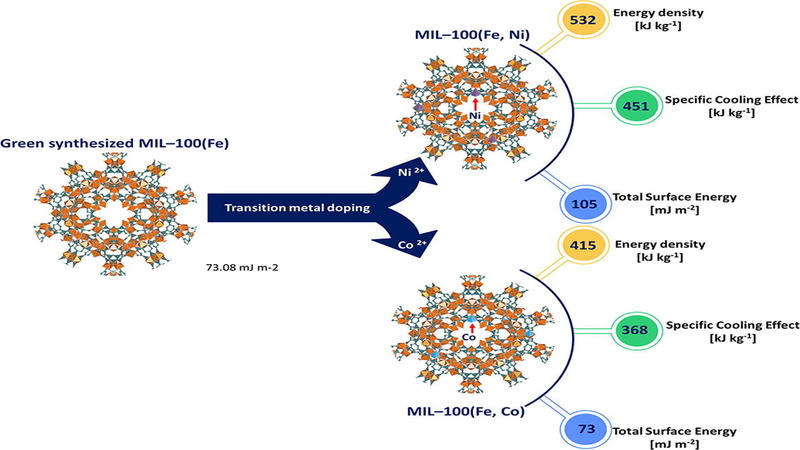
Recently metal organic frameworks (MOFs), already known as adsorbent materials, have gained increasing research interest in thermal energy storage and conversion. MOF-water working pairs have been envisaged to provide the next revolutionary advancement in the existing sorption-based thermal energy storage and conversion systems. Herein, a promising MOF for high water sorption, MIL–100(Fe), and its transition metal-doped (Ni2+ and Co2+) derivatives are produced following a green synthesis procedure. The synthesized materials were tested in water sorption measurements using a thermogravimetric technique. The experimentally obtained adsorption data were correlated with the Sun-Chakraborty adsorption isotherm model. As an example of thermal energy conversion, an adsorption chiller is considered, and hence cooling cycles were drawn using the sorption data. The cooling cycles revealed that the nickel-doped MIL–100(Fe) demonstrates the highest value of the specific cooling effect (450.79 kJ kg−1) among the studied MOFs. Inverse gas chromatography technique was employed to investigate the surface free energy of the parent and doped MIL–100(Fe) MOFs. It was found that nickel-doped MIL–100(Fe) had the highest total surface free energy of 105.41 mJ m−2. Surfaces’ Lewis acid-base activities and the work of cohesion and adhesion (towards water molecules) were investigated. The surfaces’ properties were correlated with the water adsorption isotherms, which can provide crucial information for developing optimal adsorbents targeting water as the adsorbate for an efficient and effective thermal energy storage and conversion system.